Introduction
Human homeostatic iron regulator protein (otherwise known as HFE protein) haemochromatosis (HH) is a disorder of iron overload which typically affects one in 200 persons of Northern European descent.1 Arthropathy is a well-recognised feature of HH with characteristically irreversible features. The arthritis typically involves the radial-sided finger joints, especially the index and middle metacarpophalangeal (MCP) joints, but also other small and large joints, including the hips, ankles and joints of the feet.2-5 Despite longstanding recognition of this presentation of HH, why arthropathy only affects a subset of HH patients remains unclear, thus limiting risk-factor prognostication and treatment.
Mechanisms underlying the pathophysiology of HH-related arthropathy have been proposed. Findings of iron deposition in synovial lining cells in HH patients with arthropathy indicate a potential for direct toxicity of iron to proximate joint tissues.4 Why iron is deposited and accumulates in the synovium of some but not all patients with HH is still unclear. Investigators have suggested suggest that persistently high serum ferritin concentrations may result in an increased risk of iron ‘capture’ in synovial fluid and tissue which could drive the propensity to arthropathy.2 Alternatively, given the difficulty in predicting HH-related arthropathy, even with appropriate iron management, the arthropathy may be related to alternative causal factors not directly related to iron load or high serum ferritin concentrations. Co-existent genetic factors that may promote chondral damage have been proposed as an alternative mechanism.6
The recent observation that elevated MCV segregates with arthropathy in HH has drawn attention to the relationship between iron load, as reflected by increased MCV values and the development of arthropathy.7 In this study, we have examined an independent cohort of HH participants in order to examine this observation, and also other measures of baseline iron load, to evaluate the predictive value of these measures in respect of HH joint disease, and to determine the extent and severity of the arthropathy. This study aimed to examine the relationship between iron parameters at diagnosis and the extent and severity of arthropathy in HH. A single time point cross-sectional analysis of joint structure, as determined by plain x-rays of hand and other clinically abnormal joints, was utilised. We hypothesised that the iron burden at diagnosis is a predictor of arthropathy and that arthropathy is independent of phlebotomy therapy or current iron indices.
Methods
Participants were initially recruited through a community-based public notice placed in Western Australian newspapers, which called for participants who had HH to volunteer for a medical cross-sectional study. No specific details or references to arthritis were disclosed.3 The project was approved by the South Metropolitan Human Research Ethics Committee, Western Australia. Participants were screened and a total of 19 participants with genetically confirmed C282Y HH and compatible arthropathy were included. All participants were reviewed clinically and radiologically to confirm the diagnosis of HH. Those adjudicated to most likely not have HH were excluded. Basic demographic characteristics were recorded. Baseline laboratory investigations were those available at diagnosis or determined at the time of the initial recruitment for the original cross-sectional study. Laboratory investigations, including serum iron studies and MCV at diagnosis, were recorded together with the most recent patient iron studies available at the time of data collection for the current study. These results were censored in November 2021. Due to the retrospective study design, some of the laboratory investigations were not available. To assess each patient’s arthropathy, a comprehensive evaluation was conducted by a consultant rheumatologist and a consultant radiologist with special expertise in musculoskeletal disorders as part of the original cross-sectional study.3 Both were blinded to the laboratory investigations including iron status, to reduce bias. Arthropathy evaluation included clinical and radiological assessment. Plain x-rays of the hands were available for all participants. In addition, plain x-rays of other (non-hand) clinically abnormal joints were undertaken at the time of recruitment for the initial cohort study.. Grading was undertaken according to the method of Kellgren and Lawrence.8 The finger metacarpophalangeal, radio-carpal, carpo-ulnar, elbow, shoulder, hip, knee, ankle, subtalar and intertarsal joints were included. All x-rays were read by two investigators (Breidahl and Carroll). A radiographic atlas was employed for reference and assignment of grades.8 The median interval between diagnosis of haemochromatosis and performance of x-rays was 14.5 years. A final radiological joint severity score was derived by summing the grades for all clinically involved joints in each participant, as defined above. This score was designated the summative joint damage score (SJDS). Apart from in the hands, only those joints in which clinical abnormalities were detected were x-rayed. Thus, the score is a composite score reflecting systematic radiographs of the hands and selective radiographs of joints beyond the hands found to be clinically abnormal. The prototype for this x-ray assessment has been described previously.3 The presence of chondrocalcinosis on x-ray assessment was recorded. No participants were found to have radiographic evidence of co-existent inflammatory joint disease and, hence, none required exclusion on this basis.
Statistical analysis
Descriptive statistics were employed to determine medians and inter-quartile ranges, as well as the mean values and standard deviations, where appropriate. To assess the relationship between the joint severity score and iron status, linear regression analysis using Pearson’s correlation coefficient was utilised. To determine statistical relationships between SJDS and CC, t-tests were utilised. Statistical analysis was performed using Stata (Version 16, Statacorp). Statistical significance was assigned for p<0.05.
Results
Participants with genetically confirmed C282Y homozygous HH who had arthropathy and in whom adequate baseline iron studies were available were included in this analysis. A total of 19 participants were identified who fulfilled these criteria, all of whom were Caucasian. The mean age of participants at the time of assessment was 71.9 (63 – 85) years (Table 1).

The median age at the time of diagnosis of HH was 55 (46 – 59) years. There were five females and 14 males in the study population. The median values for serum ferritin, transferrin saturation and MCV at diagnosis were 1405 (88-5760) µg/L, 84 (7-136) % and 93.50 (86-102) fL, respectively (Table 2). The mean SJDS for all participants at the time of the study was 12.57 ± 10.68. The median values for the most recent serum ferritin and transferrin saturation were 101 (21 – 790) µg/L and 58 (7-87) %, respectively.
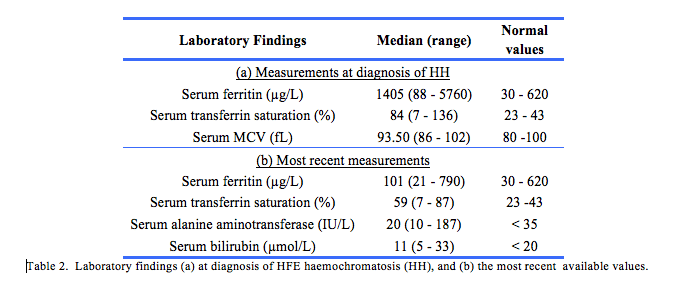
Given the relationship between liver injury and HH, liver function tests were also analysed. The median most recent alanine aminotransferase concentration was 20 (10 – 187) IU/L and bilirubin 11 (5 – 33) µmol/L. The mean time from baseline to the most recent available ferritin was 3903 days.
Chondrocalcinosis was detected in 5 of 19 (32%) of participants. SJDS were statistically higher with that condition (23.83 ± 10.38) compared to those without (7.38 ± 6.58), (t (17) = 4.22, p = 0.0005). There were no statistically significant correlations between the presence of chondrocalcinosis and MCV, transferrin saturation or ferritin concentrations.
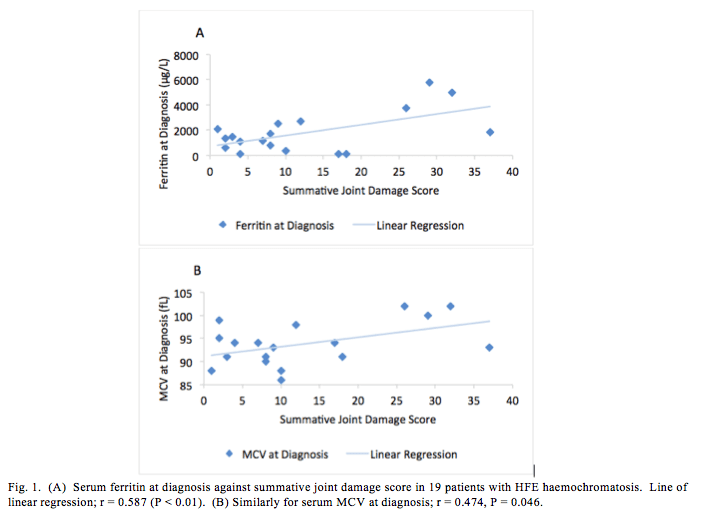
Regarding biochemical investigations at diagnosis, there was a statistically significant correlation between ferritin at diagnosis and the SJDS (Fig. 1A: r = 0.587, p = 0.01). A statistically significant correlation between MCV at diagnosis and SJDS was also observed (Fig 1B: r = 0.474, p = 0.046). No correlation was observed between transferrin saturation at diagnosis and the SJDS ( r = 0.219, p = 0.397). When the most recent ferritin and overall declines in ferritin over time were examined, no statistically significant correlation between the most recent ferritin and the SJDS was observed (r = -0.307, p = 0.284). This was despite a median decline in ferritin from diagnosis to the most recent ferritin, of 1855 ± 1688 µg/L. A correlation of borderline significance between the difference in ferritin at diagnosis and the most recent ferritin available and the SJDS was identified (r = 0.518, p = 0.0574). The median interval between diagnosis and the x-rays, upon which we relied for grading joint damage was 14.5 years (1–26 years).
Discussion
Despite major advances in respect to understanding the genetic basis, molecular biological mechanisms and overall pathophysiology of HH, the exact aetiology and mechanistic factors responsible for arthropathy in HH remain poorly understood. The findings in this study indicate that the serum ferritin concentration and MCV at diagnosis correlated with the severity of arthropathy. In contrast, neither baseline transferrin saturation, the extent of the reduction in serum ferritin over time nor the most recently available ferritin concentration correlated with structural joint damage, implying that the conventional approach to iron removal does not influence the outcome with respect to the severity of the joint disease. These findings are consistent with the long-held view that venesection does not consistently relieve musculoskeletal symptoms or prevent joint damage. Importantly, our finding that serum ferritin at diagnosis predicts the severity of arthropathy suggests that the factors contributing to the development of arthropathy appear to precede the clinical diagnosis. Thus there may be limited scope for effective prevention of joint damage thereafter. It is possible that chondrocytes expressing mutant HFE may be subject to perturbations of matrix macromolecule metabolism in the context of chronic iron overload, and excess iron may accumulate within chondrocytes in the intracellular matrix. The extent of this intracellular iron overload may be proportional to the length of time before a diagnosis is made and treatment implemented.
Though we noted a high median ferritin reduction from diagnosis to the time the most recent ferritin was determined, on average just over a decade, there was no improvement in the severity of the arthropathy, despite best practice iron depletion. Our observation regarding the association between the MCV at diagnosis and the severity of arthropathy is consistent with Rehman et al observations that MCV may act as a biomarker for the presence of HH and the propensity to develop joint tissue injury.7 Whilst the mechanisms underlying the association between MCV and HH remain unclear, it is possible that the increased MCV reflects high iron bioavailability for erythroblastogenesis and, as hypothesised above, it may also correlate with high iron concentrations in the cartilage extracellular matrix.7 Thus, higher ferritin concentrations and higher MCV at diagnosis of HH may be useful predictive markers for the development of arthropathy in HH.
Strengths of this study include the rigorous selection of participants with proven HH and characteristic iron overload, a significant proportion of whom manifested a clinically and radiologically compatible arthropathy. As far as we are aware, this is the first study to comprehensively study participants with HH arthropathy for the relationship between iron indices and musculoskeletal outcomes. Limitations include the relatively small number of participants, the incomplete data set including details of comorbidities, the retrospective nature of the data collection (historic laboratory results), limited imaging data as only clinically abnormal joints were imaged, and the limited imaging modalities available (plain films, but not ultrasound, computer tomography or magnetic resonance imaging). Furthermore, as this was not a prospective protocol driven study, the timing of x-rays after diagnosis was not fixed and varied considerably, reflecting current clinical practice. This diversity emphasises the sometimes very long interval between diagnosis of iron overload and the onset of or recognition of musculoskeletal symptoms sufficient to prompt imaging.
Larger prospective studies, preferably incorporating higher numbers of females and designed to assess with greater rigour potential gender differences as well as the predictive properties of serum ferritin concentrations at diagnosis and prior to phlebotomy, are likely to be informative. In addition, further examination of baseline MCV and transferrin saturations with reference to the timing, pattern and severity of arthropathy, appears warranted. The higher than expected frequency of chondrocalcinosis in this cohort and the trend towards higher SJDS in that group needs to be assessed further.
Newer chelating agents may have a capacity to limit the accumulation of iron in joint tissues, including the extracellular cartilage matrix, and thus confer a better joint outcome. The absence of a reliable serum biomarker for joint damage is a significant limiting factor in the evaluation of current and new therapies. To our knowledge, no iron chelators have been tested for potential impact on the evolution of arthropathy in HFE haemochromatosis. Imaging remains the gold standard. A moderate-term (say, over 3–5 years) early intervention trial comparing combined use of chelators and venesection with venesection alone and with particular reference to radiological outcomes in commonly affected joints may be informative.
Conclusion
In genetically proven C282Y homozygous HH arthropathy, serum ferritin and MCV at diagnosis of HH were found to correlate positively with the severity of arthropathy, whereas neither the initial transferrin saturation nor the extent to which iron removal reduces ferritin concentrations, were found to be related to joint outcome. Similarly, arthropathy develops and progresses despite current best practice treatment for HH, further emphasising that systemic “de-ironing” appears to be insufficient to either prevent or substantially restrict joint damage and, in turn, joint dysfunction. This is an important finding which has the potential to inform prognosis and assist in the development of a better understanding of mechanistic factors responsible for chondral resorption and joint failure. Comprehending these factors remains key to the implementation of more effective preventative strategies and, in turn, better joint preservation and improved musculoskeletal outcomes.
Provenance: Externally reviewed
Ethical approval: Study approved by the South Metropolitan Human Research Ethics Committee, Perth.
Disclosures: None
Acknowledgements: None
Corresponding author: Dr James Chen, Department of Gastroenterology & Hepatology, Fiona Stanley Hospital, Murdoch, Western Australia 6150, Australia. Email: james.chen@health.wa.gov.au









