INTRODUCTION
Allogeneic haemopoietic progenitor cell transplantation (alloHPCT) involves the infusion of third party-derived haemopoietic precursor cells into a recipient who has undergone sufficient chemotherapy or radiotherapy conditioning treatment to allow engraftment of these cells and eventually full haemopoietic and immunological reconstitution. The treatment is effective as replacement of haemopoiesis in bone marrow failure syndromes, and allows high doses of chemotherapy as well as life-long immunological surveillance against relapse to treat malignant conditions. Donors are preferentially siblings who are human leukocyte antigen (HLA) matched at both class I and II loci, HLA-matched unrelated donors, or alternative donors such as partially matched unrelated adult, related adult or cryopreserved umbilical cord blood products. In usual circumstances, donor cells are infused ‘fresh’, immediately after collection, into a recipient who has just received conditioning therapy. Risks to recipients include toxicity of chemotherapy or radiotherapy, infection during aplasia or immunological reconstitution, relapse and graft-versus-host disease.
Western Australia (WA) has a population of 2.7 million (December 2019) and more than 80 hospitals spread across 2.5 million square kilometers (1, 2). The wide catchment area poses a challenge in providing timely and equitable healthcare to the population. A new flagship tertiary metropolitan hospital (Fiona Stanley Hospital (FSH)) with 783 beds was opened in February 2015(3). The Bone Marrow Transplantation Programme (BMTP) at FSH provides a central state-wide service for all adult alloHPCT in WA. Transitioning the service from pre-existing tertiary hospitals and creating a new centre to cater for increasing demands can be challenging. This study aimed to assess the service delivery and outcomes of patients undergoing alloHPCT at FSH since the opening.
MATERIAL AND METHODS
Patient selection and characteristics
We retrospectively analysed consecutive 193 adult patients having alloHPCT under the BMTP at FSH, from 1 February 2015 to 31 December 2019. Exclusion criteria included syngeneic transplants (n = 1) and patients who underwent a second alloHPCT during the study period (n = 1). The total number of alloHPCT per year in Western Australia since 2007 was also recorded. Ethics approval for data collection was granted by FSH Department of Safety, Quality and Risk, and all patients signed informed consent for the collection of their data for research purposes.
Patient characteristics collected included age of recipient at the time of transplantation, sex, Karnofsky performance status, and pre-transplant disease characteristics (diagnosis type, date of diagnosis and disease status). Transplant characteristics included number of previous alloHPCT, type of conditioning, graft source and donor match, use of anti-thymocyte globulin (ATG) and GVHD prophylaxis. The Haemopoietic Comorbidity Index (HCT-CI), which incorporates pre-transplant clinical history, pulmonary function, liver and renal function results to determine risk of transplant-related mortality, and the revised Disease Risk Indices (DRI), which groups haematological malignancies according to relapse risk, were calculated according to published sources (4, 5).
Acute graft-versus-host disease (aGVHD) was graded according to according to published consensus criteria, and chronic graft-versus-host disease (cGVHD) as classified according to revised 2014 NIH criteria (6, 7). The cumulative incidence of aGVHD grade II to grade IV at 6 months and aGVHD grade III to IV at 6 months were analysed. For cGVHD, only patients who survived more than 100 days were included in the analysis. Patients who had alloHPCT from 1 July 2019 were excluded to allow for sufficient clinical data being available for at least 6 months follow up. The cumulative incidence of cGVHD of any severity, moderate or severe, and of severe only after 2 years of follow-up, were analysed. Immunosuppression was defined as at least 5mg prednisolone or equivalent, or other systemic immunosuppressive agent at any dose.
Neutrophil engraftment was defined as the first day of absolute neutrophil count ≥0.5 x 109/L for at least 3 consecutive days. Platelet engraftment was defined as the first day of platelet count ≥20 x 109/L for at least 7 days without platelet transfusions.
Statistical analysis
The median and range was calculated for continuous variables. The probabilities of overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. Probabilities of aGVHD, cGVHD, transplant-related mortality (TRM) and relapse were calculated using cumulative incidence analysis to accommodate competing risks (8). Death without aGVHD or cGVHD was the competing risk for its respective cumulative incidence. Relapse was used as competing risk for TRM and death without disease relapse as the competing risk for relapse. Ninety-five percent confidence intervals for probabilities were derived from point-wise estimates and were calculated using standard techniques. Statistical analyses were performed using SPSS® statistical software (version 23; Chicago, Il, USA), GraphPad Prism (version 8.4.3; La Jolla, Ca, USA) and R (version 4.0.2; cran.r-project.org) on the RStudio platform (version 1.3.1056; Boston, Ma, USA).
RESULTS
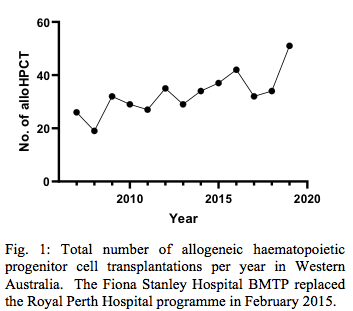
The total annual number of patients undergoing alloHPCT in WA has been increasing progressively since 2007 (Fig. 1), and reached 54 patients in 2019.
Patient, disease, and transplant-related characteristics
The characteristics of patients who had alloHPCT are summarised in Table 1. Median age at transplant was 51 y (range, 16 – 67 y) and 60.1% were males. Acute myeloid leukaemia was the most common indication (42.5%), followed by myelodysplastic syndromes (16.1%). Median time from diagnosis to alloHPCT (all patients) was 9.4 months (range, 2.0 – 301.2 [one patient was diagnosed in 1992]). In patients with AML who were in their first complete remission the median time from diagnosis to alloHPCT was 5.7 m (range, 2.4 – 28.4 m). The corresponding datum for patients with acute lymphoblastic leukaemia (ALL) in CR1 was 8.6 m (range, 4.6 – 12.6 m).

Referrals for AML in the most recent two years of the study period were analysed to reveal patterns of referral and transplantation. Of 41 referrals, 27 (65.9%) received alloHSCT, 26.8% were deemed not suitable or declined transplant, and 7.3% died before transplant. Median time from referral to review in transplant clinic was 26 days (range 7-105 days) and did not differ according to referring centre (p = 0.534).

The majority of patients (64.2%) received transplants from unrelated donors. Peripheral blood was the most common graft source (90.7%). Reduced intensity conditioning (RIC) was used in 49.7% of all recipients. A minority (n = 29, 15%) received anti-thymocyte globulin. Cyclosporin combined with methotrexate was the most frequently used prophylaxis for graft-versus-host disease (93.3%).
The baseline Karnofsky performance status of the cohort at time of transplant was ≥ 90% in 76.7% of patients. Eleven patients did not have performance status recorded. The pre-transplant HCT-CI score was 0 in 28% of patients, 1-2 in 14.5%, and 3 or higher in 17.6% of patients. HCT-CI was not recorded in 39.9% of patients, and was more likely to be missing for patients undergoing transplant during the early years of the programme (30 in 2016 vs. 0 in 2019). The pre-transplant refined DRI score was ‘Low’ in 8.8%, ‘Intermediate’ in 64.2%, ‘High’ in 19.7% and ‘Very High’ in 4.1% of the cohort. Six patients were unclassifiable as their pre-transplant disease was aplastic anaemia.
GVHD incidence
The graft-versus-host disease outcomes are summarised in Table 2. Overall, 101 patients were diagnosed with aGVHD of any grade and 92 patients had no aGVHD. The cumulative incidence probabilities are outlined on Fig. 2. The cumulative incidence of grade II to IV aGVHD at 6 months was 35.4% (95% CI 28.4 – 42.4%) (Fig. 2a). The cumulative incidence of grade III or IV aGVHD at 6 m was 10.4% (95% CI 6.5 – 15.4%) (Fig. 2b).
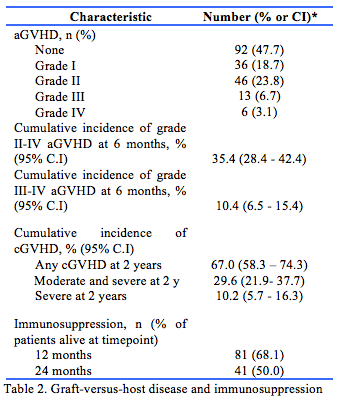
In patients who survived >100 days after alloHPCT and had transplant between 1 February 2015 to 30 June 2019 (n = 147), the cumulative incidence of cGVHD of any severity at 2 years was 67.0% (95% CI 58.3 – 74.3%). The cumulative incidence of moderate or severe cGVHD at 2 years was 29.6% (95% CI 21.9 – 37.7%) (Fig. 2c). The cumulative incidence of severe cGVHD at 2 years was 10.2% (95% CI 5.7 – 16.3%) (Fig. 2d).
In survivors 12 and 24 m after alloHPCT, 68.1% and 50.0% of patients remained on immunosuppression.
Engraftment and survival outcomes
Clinical outcomes and survival end-points are described on Table 3. Median follow-up was 24.3 m (range, 5.9 – 62.1 m). The median time to neutrophil engraftment was 16 days (range, 6 – 36 days) and 94.8% of patients achieved neutrophil recovery. Median time to platelet engraftment was 18 days (range, 6 – 136 days) and 88.6% achieved platelet recovery.
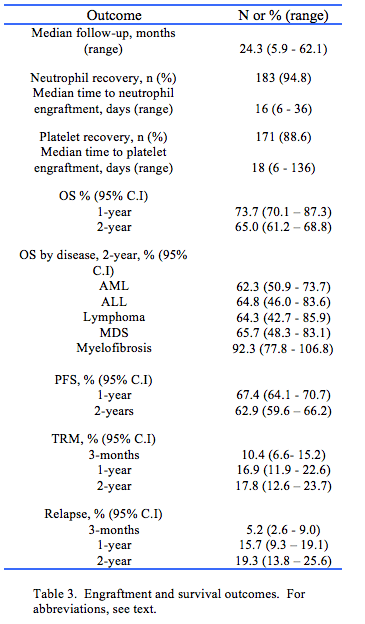
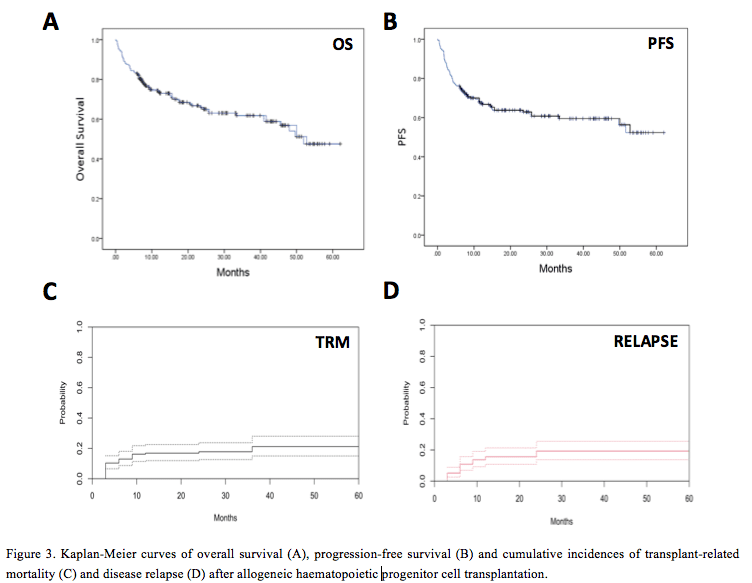
The estimated OS for the entire cohort at 1 year was 73.7% (95% CI 70.1 – 77.3%) and the 2-year OS was 65.0% (95% CI 61.2 – 68.8%) (Fig. 3a). The estimated PFS at 1-year was 67.4% (95% CI 64.1 – 70.7%) and PFS at 2-years was 62.9% (95% CI 59.6 – 66.2%) (Fig. 3b). The 2-year cumulative incidence of TRM was 17.8% (95% CI 12.6 – 23.7%) following alloHPCT (Fig. 3c) and was similar for both RIC and myeloablative conditioning (MAC) recipients (p = 0.622). The 2-year cumulative incidence of disease relapse was 19.3% (95% CI 13.8 – 25.6%) (Fig. 3d).
DISCUSSION
We have analysed the five-year outcomes of adult patients undergoing alloHPCT in Western Australia for both non-malignant and malignant indications. The overall increase in the total number of patients undergoing alloHPCT since 2007 is likely to be a combination of reflection of demands of a growing population and also growing confidence by the authors in offering alloHPCT to older patients.
Our results compare well with other Australian centres (1-year survival, 73.7% for all donor types) compared to the survival reported by the Australian Bone Marrow Transplant Recipient Registry (63 – 70%). (9). Our study reveals a 1-year TRM of 16.9% (all donor type) compared to 19.7% in unrelated alloHPCT (9).
These comparisons, while appearing favourable, are limited by a lack of adjustment for patient and disease risk factors which may not be equal across groups. Such benchmarking practices are performed regularly by the Australian Bone Marrow Transplant Recipient Registry, but not yet for the FSH 2015-2019 cohort. A number of developments may account for the reassuring outcomes observed at FSH. First, incremental improvements in donor selection and supportive care have led to improved outcomes at other centres over recent decades, and current FSH practice has benefitted from these improvements (10, 11). Second, FSH BMTP embarked on a rigorous quality management programme from its establishment, leading to full accreditation by the National Australian Testing Authority (NATA) in 2016 and the Foundation for the Accreditation of Cellular Therapy (FACT) in 2019. These achievements reflect the development of policies and procedures for audit, reporting of adverse events and near-misses, practice improvement, outcome reporting and clinical practice, as well as the development of a close multi-disciplinary quality management team. Indeed, in 2016, the FSH BMTP created 38 new clinical standard operating procedures alone. Such quality management procedures have been shown to improve patient outcomes after transplantation at other centres (12-14). Finally, FSH BMTP has benefitted from increasing involvement in clinical trials in cellular therapy. In 2015, only one interventional clinical trial was available to alloHPCT recipients at FSH, and even then only for the minority who developed grade II-IV aGVHD. By contrast, five prospective interventional clinical trials were available for the vast majority of patients undergoing allogeneic transplantation in 2019.
The most common indication for alloHPCT was AML. The median wait time from diagnosis to transplant (5.7 months) in those being transplanted in first remission appears relatively long, given that the decision to proceed to transplant is usually made within the first 21 days after diagnosis, and it is known that further delay after achieving remission risks either relapse or toxicity from ongoing consolidation chemotherapy. Efforts to shorten the delay until alloHPCT appear warranted, to be achieved via increased early communication with referring centres, optimising donor search and streamlining clinical review and workup prior to transplant.
Nearly two thirds (64.2%) of recipients in Western Australia received HPCT from an unrelated donor, a marked reversal of early transplantation practice where almost all donors were matched siblings. This likely reflects both smaller family sizes, resulting in lower likelihood of identifying a matched related donor, and the increased size of the unrelated donor registry, now numbering nearly 37 million donors worldwide, most of whom reside in Europe or the United States of America (15). This reliance on unrelated donors has created significant challenges during the current COVID-19 pandemic, due to reduced availability of donors and severe disruption in international travel for HPC products. Previously, such products were collected locally and shipped to FSH accompanied by a courier, to be infused immediately on arrival. This practice has become untenable and unsafe in the current environment, and all unrelated donor cells are now being cryopreserved prior to shipment, and recipient conditioning therapy is not started until the cryopreserved product has arrived at FSH and product viability is established. While these practices have enabled some unrelated donor transplants to continue, they are logistically complex and do not overcome the problem of reduced donor availability due to local hospital incapacity, donor illness or unwillingness to attend medical facilities for collection. These circumstances have led to increased alternative donor use at FSH in 2020. However, the allogeneic transplant service is still active in Western Australia compared to other interstate and international transplant centres where services had to be halted due to increasing numbers of COVID-19 cases.
In keeping with rapid haematological advance in this area, the most recent clinical standard operating procedures at BMTP include quality processes for delivering chimeric antigen receptor T-cell therapy (CAR-T). BMTP increasingly participates in national and international clinical trials, and in 2020 successfully delivered the first CAR-T treatment. We anticipate that BMTP will become further involved in immune effector cell therapies and therapeutic clinical trials. This study provides details of patient disease characteristics, pre-transplant function status, transplant characteristics and clinical outcomes in patients undergoing alloHPCT in Western Australia. These clinical details have not previously been published. The FSH BMTP has had results that are comparable to published national data.
Provenance: Externally reviewed
Ethical Approval: Granted by Fiona Stanley Hospital Department of Safety, Quality and Risk.
Disclosures: None
Acknowledgments: The authors are grateful for the expertise and diligence of the haematologists, junior doctors, nurses, scientists and allied health staff who form the wider BMTP team at FSH.
Corresponding author: Dr Duncan Purtill, Department of Haematology, Fiona Stanley Hospital, Murdoch WA 6150 Australia. Email: duncan.purtill@health.wa.gov.au









