Introduction
Adherence to guidelines for thromboprophylaxis in medical patients (MT) using LMWH has been shown repeatedly to be poor.1-6 Improvement is possible using educational and marketing techniques, changing prescribing chart design, adding prescriber support, and repeated auditing, but this begs two questions, namely the reasons for poor adherence and the quality of the guidelines to which adherence is required. MT guidelines in Australia and overseas have been criticised.7-10 Expressions of regret over non-adherence and entreaties to avoid it imply that MT is a good with no major adverse effects. In fact, LMWH has a significant bleeding risk.11-13 Furthermore, the purported benefit of prophylaxis has not been demonstrated directly. Rather, it relies on extrapolation from clinical trials powered to detect differences in sub-clinical venous thromboembolism (VTE) events.14 Our previous findings,15 based on modelling data from the Prevention of Recurrent Venous Thromboembolism (PREVENT) trial,16 suggested that though MT lowers VTE rates, the benefit is offset by a possible increase in mortality from bleeding. This was reinforced in a separate study17 based on a Cochrane Collaboration meta-analysis.11
The assumption that non-adherence means loss of health benefits is valid only if guidelines are soundly based. If this condition is not satisfied, non-adherence may have mixed effects or may even protect patients from harm. The aim of this study was to estimate the performance of current and proposed guidelines for MT using our existing model of thromboprophylaxis,15 duly modified to provide for non-adherence.
Methods
Estimates were based on a decision tree model of new inpatient thrombotic events (DVT, fatal or non-fatal PE) and post-thrombotic syndrome) as well as fatal and non-fatal major bleeds,15 at the rates found in the PREVENT trial.16 The model was applied to medical patients admitted annually to public and private hospitals in Australia (index year 2011-2012). Of several possible candidates, the PREVENT trial was selected because of its unique design, according to which the natural history of thrombosis was maintained because no routine imaging was undertaken up to the 14th day of admission. As a result, and unlike other MT trials, no treatment was given for sub-clinical thrombotic events up to that time point. The model also contained Australian dollar costs of hospitalisation, prophylaxis and treatment of thrombotic and haemorrhagic events, as previously detailed.15 Patient populations were studied under three guideline categories containing 4 separate guidelines, consisting of a broad category equivalent to current UK18 and Australian19,20 guidelines, and two further categories with progressively restricted eligibility, namely the guidelines of the American College of Chest Physicians21 and those proposed previously by the author.22 The baseline VTE risk increased progressively in each category, as weaker risk factors are excluded. Categories are referred to in this paper numerically (1 to 3) or by the percentage of the inpatient population rendered eligible by each guideline (table 1).
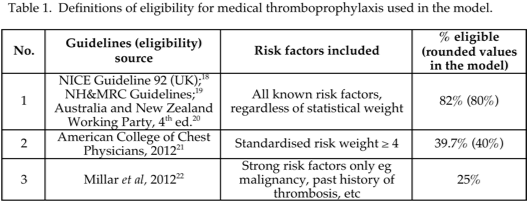
Patients eligible for MT under each guideline were divided into two subgroups. One received LMWH represented by enoxaparin 40 mg daily (adherent), the other did not (non-adherent). Enoxaparin is the only LMWH registered for MT in Australia. The model estimated the pattern of clinical outcomes as above and Australian dollar costs at full adherence, and at non-adherence rates from 10% to 75% and in some analyses 60%, a rounded value from that reported (58%) in the ENDORSE (Epidemiologic International Day for the Evaluation of Patients at risk for Venous Thrombo-embolism in the Acute Hospital Care Setting) study.1 The non-adherent groups were assumed to lose the PREVENT trial-based relative risk reduction for DVT and PE, and to suffer these events at the relevant attributable baseline risk. The anticoagulant-related bleeding risk was assumed to be constant. Model outputs were compared to results at full adherence and where relevant to those obtained with no prophylaxis.
As it exists as a theoretical possibility, I also studied “reverse non-adherence”, defined as administration of LMWH to non-eligible patients. These patients, who are by definition at low VTE risk, were assumed to obtain the relative risk reduction for non-fatal VTE events documented in the PREVENT study, but were exposed to the reported major bleeding risk. Minor bleeding was ignored. As it appeared unlikely that reverse non-adherence would be as common as non-adherence, the range over which it was studied was limited to 40% and below. The combination of reverse non-adherence at an incidence of 20% and non-adherence at 60% was also studied.
The PREVENT trial reported higher than average values for major bleeds and case fatality rates for PE and haemorrhage, compared to similar trials and epidemiological studies respectively. As these findings may be a source of bias, additional estimates based on (a) the mean LMWH-related major bleeding rate (active – placebo rates) reported by the Cochrane Collaboration11 and (b) case fatality rates as above found in independent epidemiological studies,23-26 were made. Formal sensitivity analysis was not performed because the relative effect of non-adherence is similar for all sensitivity assumptions. To permit direct comparison with our previous publication, patient numbers, unit costs and the value of the Australian dollar were maintained at 2012 values.
This study analysis was conducted using clinical trial, epidemiological and cost data available in the public domain. It did not use individual patient data. Ethics approval was not required.
Results
As previously reported,15 the base-case model outputs without non-adherence reported a benefit of MT on non-fatal VTE events, fatal PE and post-thrombotic syndrome compared to no prophylaxis. These benefits were offset by the clinical and economic detriment of major bleeds and deaths from bleeding. Progressive restriction of eligibility for MT to patients with stronger risk factors diminished the VTE benefits to a modest extent but reduced the clinical and cost penalties arising from bleeding by virtue of the decline in the number of treated patients.
Non-adherence
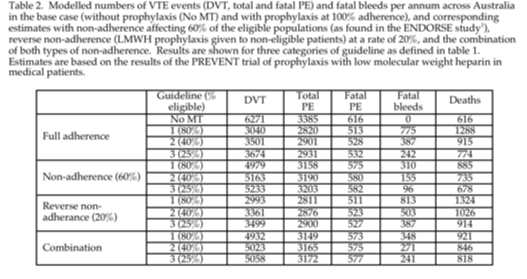
As predicted, eligible patients in each guideline category who were not given prophylaxis lost clinical VTE benefits, including reduced post-thrombotic syndrome (data not shown), according to the degree of non-adherence. They also obtained an offsetting benefit from reduced risk of major bleeds (fig. 1). These reciprocal trends were related to the level of eligibility imposed under each guideline type (fig. 1), and are reflected in the mortality data shown for 60% non-adherence (table 2) and across the studied range of non-adherence (figs 1 and 2). Figure 2 also demonstrates the greater impact of broad guidelines on mortality and the greater numbers of lives saved by non-adherence. The net effect of all degrees of non-adherence was to increase non-fatal VTE rates while decreasing overall mortality.
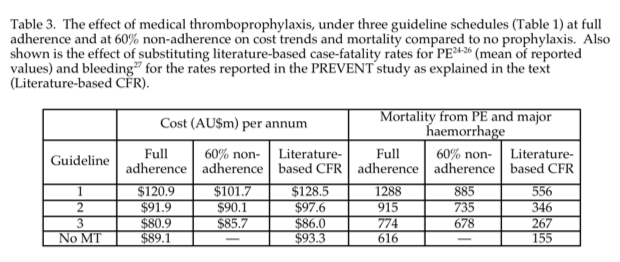
Variation in total cost with non-adherence was also related to the guideline type (Table 3). Non-adherence reduced the cost of MT guidelines with broad eligibility because of reduced costs in treating major haemorrhage, whereas costs increased modestly with restrictive guidelines, in that case due to increased costs of treating non-fatal PE.
Reverse non-adherence
Reverse non-adherence decreased VTE incidence modestly because the baseline risk in non-eligible patients is low. The risk of major bleeding and mortality increased in proportion to the number of ineligible patients treated (table 2) and this was the dominant effect. The trend is more marked as eligibility for MT is restricted, because the greater number of non-eligible patients.
When both types of non-adherence were combined, the bleeding benefit associated with non-adherence was offset by bleeding caused by reverse non-adherence, and there was a minor added benefit for non-fatal VTE events. Again, the pattern varied according to the category of guideline. (table 2).
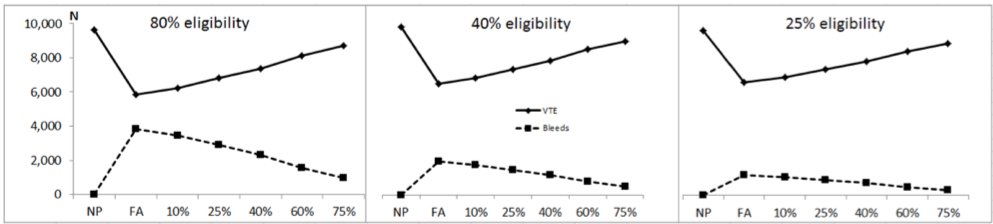
Fig. 1. Modelled effect of non-adherence with recommended medical prophylaxis at rates of 10% to 75% on total venous thromboembolism (VTE – DVT plus non-fatal PE) (solid lines) and major bleeds (dotted lines). Also shown are estimated values with no prophylaxis (NP) and with prophylaxis at full adherence (FA). Each panel represents a different guideline for MT based on selection of risk factors for venous thromboembolism (left hand panel, 80% eligibility equivalent to current UK18 and Australian19,20 guidelines; centre panel, 40% eligibility, equivalent to US guidelines;21 right hand panel, 25% eligibility, as previously suggested by the author22). Estimates were derived from the results of the PREVENT study as previously described.
Effect of incorporating epidemiological values for case fatality rates into the model
PE and major bleeding in the model are shown for the PREVENT study values, with and without non-adherence at a rate of 60% (Fig. 3), and the effect of substituting literature CFR values for PE and major bleeding (Table 3, Fig. 3) Fatalities diminished with the substituted values because they were lower than reported by PREVENT, but for the same reason prophylaxis saved fewer lives, and the incidence of non-fatal PE increased. Thus the paradoxical modelled mortality detriment of MT and the benefit of non-adherence remained, but at a lower level when literature values were used. This was also the pattern when the mean major bleeding rate reported by the Cochrane Collaboration11 was used in the model, and when all adjustments were applied concurrently (Fig. 3).
Discussion
This study confirms the expectation that non-adherence with MT has mixed effects corresponding to the known beneficial and adverse actions of LMWH shown in all clinical trials, but based especially on the PREVENT trial data.
If MT had no adverse effects, the only justifications for deliberate non-adherence would be that the benefits are trivial or costs are excessive, or both. Neither applies to MT, though at the incidence of clinical VTE events found in general medical patients (0.146%27 to 1.58%;28 with most reports clustered around 0.5%) the absolute benefit is low. In practice, however, the situation is more complex. A recent Cochrane Collaboration meta-analysis reported that DVT prophylaxis in medical patients is associated with a significant risk of major haemorrhage,11 in spite of exclusion, from all trials, of patients with risk factors for bleeding. It follows that unless the case-fatality rate for major bleeding is zero, which is unlikely, MT has a mortality detriment. Thus MT entails both benefit and hazard. Where both a detriment and a benefit derive from a medical activity, the effect of non-adherence will be to negate both. In the case of MT this means a reduction in the non-fatal VTE benefit but also a reduction in the anticoagulant major bleeding risk and associated mortality. The present study is in keeping with this. I also demonstrate here, and have argued previously on general grounds9 supported by modelling,15 that the detriment and costs will be reduced, and the benefit to hazard ratio improved, if eligibility for MT is restricted to patients with statistically strong risk factors. Current MT guidelines in Australia and the UK entail greater detriment than alternatives involving more stringent patient selection, such as the guideline of the American College of Chest Physicians21 (which requires a grading based on risk factor weight) and the author’s proposed eligibility criteria22 (which restrict eligibility to patients who have only the strongest known risk factors. Restrictive guidelines lessen unnecessary exposure of patients at relatively low VTE risk to the risk of major bleeding that is intrinsic to the use of anticoagulant drugs.
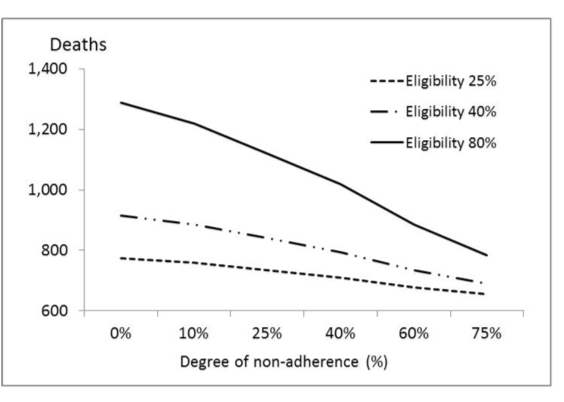
Fig. 2. Estimated deaths from pulmonary embolus plus major bleeds in medical patients across Australia per annum in the course of medical thromboprophylaxis given at full adherence under each of three thromboprophylaxis guideline as defined in table 1, and in the presence of non-adherence to the guidelines at rates from 0% (i.e. full adherence) to 75% non-adherence).
Reverse non-adherence is a second possible pattern of departure from good practice. Though it seems substantially less likely that prescribers will deliberately apply prophylaxis in non-eligible patients, several circumstances may promote it. First, it will be present by definition if a mistaken policy of giving MT to all patients without a bleeding risk is adopted. This is not advocated in any guidelines but may result from uncritical or oversimplified prophylaxis practice. Second, hospital prescribing is often undertaken by relatively junior medical staff. Third, if guidelines include weak risk factors to define “high risk” patients, the application of prophylaxis may in fact be equivalent to reverse non-adherence, at least for patients without stronger risk factors. One such factor is advanced age (expressed in guidelines as age greater than a specified value). We have pointed out previously that there is no sound basis for including this apparent risk factor, as its significance in epidemiological studies and randomised trials disappears on multivariate analysis.29,30 Current Australian guidelines, which contain the risk factors “age > 60” or “age > 40” respectively, are of this type.19,20 Including such a risk factor inevitably causes high eligibility rates because the mean age of general medical inpatients in Australia is over 70 years. Broad eligibility criteria are misguided because they cause inclusion of patients who have little to gain in decreased VTE but are exposed to the full bleeding risk of prophylaxis.
This study has several limitations. As with any modelling, the outcomes depend on assumptions within the model, in this case, that the results of the PREVENT trial are a sound basis for a modelling process that involves extrapolation to the entire Australian medical inpatient population, and that the results of the trial are in fact reliable. PREVENT was the largest randomised trial of MT. However, it was underpowered for clinical VTE events, which were not significantly different in active and placebo-treated groups. These limitations have been addressed previously.15 However, the proposed double jeopardy of non-adherence depends on only two suppositions. First, that MT reduces VTE event rates (based on extrapolation from trials of sub-clinical events). Second, that it also causes severe bleeding. Both suppositions receive literature support. Indeed, the first is the justification for MT in spite of the absence of direct trial evidence of clinical event reduction. The second has been found in all trials, though at varying rates.11-13 Thus non-adherence to MT guidelines must produce mixed outcomes. The purpose of selecting the PREVENT trial data for modelling purposes is to obtain the scale of likely effects of MT, given that it is the largest published trial, and to ensure consistency with the natural history of clinical thrombosis in medical patients, in whom routine imaging to detect sub-clinical thrombosis is not the norm. This is not possible using data from any other trial because LMWH was given to treat subclinical events. Nevertheless, sole reliance on the PREVENT trial may cause overstatement of the mortality risk of MT because of the high bleeding rates compared to other trials, and the apparently high case fatality rates for both PE and major haemorrhage. Because of the above limitations, the importsance of this study lies in hypothesis generation. Nevertheless, it is undeniable that non-adherence with a medical activity has opposing effects when both benefit and hazard exist, as appears to be the case for MT.
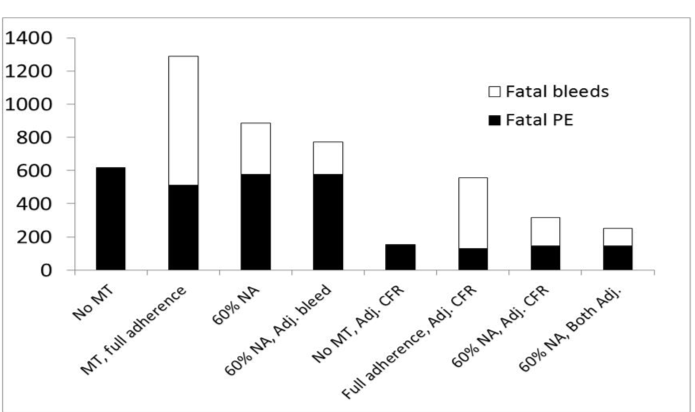
Fig. 3. Modelled deaths from PE and major haemorrhage under guideline category 1, as currently applies in the United Kingdom18 and Australia,19,20 showing the effect of changing the PREVENT value of major bleeding incidence to the mean value reported in a Cochrane Collaboration meta-analysis, the values for case fatality rates for pulmonary embolus and major bleeds as described under methods, and both changes together. Changing PE case-fatality rates also affects the estimates of mortality on the comparators of no prophylaxis and thromboprophylaxis at full adherence, as shown. Prophylaxis increased mortality under all assumptions, and mortality improved with non-adherence. MT, medical thromboprophylaxis; NA, non-adherence with prophylaxis; Adj. CFR, adjusted case-fatality rates used in the model; Adj. bleed, adjusted major bleeding incidence; Both Adj., adjusted bleeding incidence and case-fatality rates combined.









