INTRODUCTION
High-flow nasal oxygen (HFNO) and non-invasive ventilation (NIV) may reduce the requirement for invasive ventilation in selected patients with respiratory failure. However, there is concern that, when used in the treatment of coronavirus disease 2019 (COVID-19), both modalities may increase the risk of transmission to healthcare workers (HCW) through small droplet aerosol generation. Small droplet aerosols have the potential to remain airborne for extended periods of time, follow air currents within the room, and penetrate deep into the airways, facilitating airborne viral transmission.1 Uncertainty about the relative risk of aerosol-generating procedures (AGP) has contributed to strikingly different recommendations amongst international guidelines on their use. Much of the evidence concerning use of NIV and HFNO in COVID-19 patients come from experiences with two previous coronavirus outbreaks, the 2003 Severe Acute Respiratory Syndrome (SARS) epidemic and the 2012 Middle Eastern Respiratory Syndrome (MERS) epidemic.
The potential for airborne transmission of a pathogen is influenced by a variety of droplet-related, pathogen-related, host-related, and environmental factors.1-3 A recent study showed that SARS-CoV-2 (the virus which causes COVID-19) has similar aerosol and surface stability to SARS-CoV-1 (which causes SARS), with a median half-life of 1.1-1.2 hours in generated aerosols (droplet diameter <5 µm) and has sustained viability in aerosols throughout the duration of a three-hour experiment.4 The stability of SARS-CoV-2 on a variety of surfaces, including plastic, stainless steel, and cardboard, was also similar to that of SARS-CoV-1.4 These characteristics of SARS-CoV-2, coupled with previous evidence of SARS-CoV-1 airborne transmission,5 make it plausible that SARS-CoV-2 may be transmitted at least in part via small particle aerosols. This is concerning for HCW, who are at risk of contracting the virus while caring for patients, especially during AGP. Currently, there is a lack of consensus on the relative safety of HFNO and NIV use in patients with COVID-19. The optimal delivery of ventilatory support to COVID-19 patients must be balanced with any risks to HCW. A recent systematic review by Agarwal et al6 examining the efficacy and safety of HFNO in COVID-19 concluded that the risk of airborne transmission of SARS-CoV-2 to HCW during HFNO therapy is uncertain. We carried out a systematic review of the aerosolisation risks of SARS-CoV-2 during HFNO and NIV and the associated risk to HCW of contracting COVID-19.
METHODS
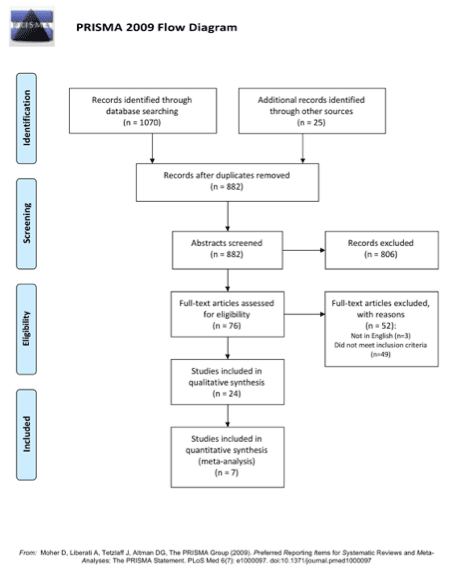
The study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1).7
Search Strategy
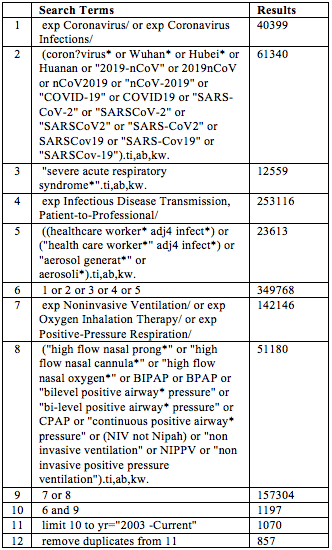
The Ovid MEDLINE and Embase databases were searched for the period 1/1/2003 to 22/5/2020, for published literature assessing HCW safety with use of HFNO or NIV. A hand-search of the reference lists of key articles was also conducted. The search strategy included the broad terms: ‘high-flow nasal oxygen’ OR ‘non-invasive ventilation’ AND ‘COVID-19’. The full list of search terms is shown in Table 1. Two authors (TP and HN) independently searched, screened and determined study eligibility. Any disagreement was resolved by a third author (EL). Study quality was assessed using the GRADE criteria for study evidence and Cochrane Collaboration’s tool for assessing risk of bias.8 A targeted search of guidelines from government and non-government organisations pertaining to the use of HFNO and NIV use in COVID-19 was also conducted.
Eligibility criteria
Eligible literature included published journal articles published in English. Articles discussing risk of transmission to HCW after exposure to HFNO or NIV, or those that discussed HFNO or NIV in relation to HCW safety, were included. Guidelines, published letters, and reviews were also examined for background information. Reference lists of these identified works were hand-searched for relevant studies.
Data collection
A coding sheet was piloted by two authors (HN and TP). Study data were extracted from eligible studies independently, and then discussed between two authors (HN and TP). The final author (EL) oversaw this process to ensure fidelity and to adjudicate any disagreements. Study characteristics were discussed, where available, including the role of relevant HCW reported in the study (physicians, nurses, physiotherapists, etc.), type of test used to confirm infection (including laboratory testing such as RT-PCR or serology, or radiological evidence of infection), personal protective equipment used, and type of intervention HCW were exposed to, including HFNO and NIV. Data for quantitative analysis included number of HCW exposed and number of patients infected with COVID-19. The number of HCW infected according to their exposure was also recorded. The outcome of interest was the relative risk (RR) of HCW infection after exposure to patients with confirmed coronavirus infection receiving NIV or HFNO.
Statistical analysis
Relevant studies were pooled for quantitative analysis, using a DerSimonian and Laird Random Effects Model.9 Two by two contingency tables were constructed in Microsoft Excel (Version 16.37, 2020) for summary estimate analysis. Statistical analysis was performed using these tables in Microsoft Excel. Fisher’s Exact Test was used for significance where table values were less than 5. Chi-squared analysis was conducted where table values exceeded this. A forest plot was constructed using the DistillerSR Forest Plot Generator from Evidence Partners.10 It was expected that there would be significant heterogeneity in the studies’ outcome measures. Where relevant, an inductive approach to thematic analysis of qualitative data based on the phases described by Braun and Clarke11 was undertaken to summarise articles.
RESULTS
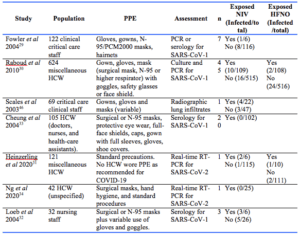
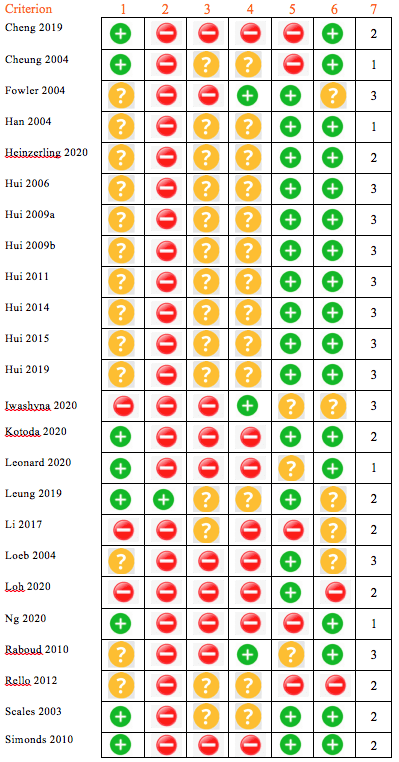
The literature search identified 1095 potentially relevant articles. Of these, 24 trials were included in the systematic review. The PRISMA flowchart for article identification is illustrated in Fig. 1. Trials were published between 2003 and 2020. They were conducted in Asia (n=13), North America (n=7), Europe (n=1), United Kingdom (n=1) and Singapore (n=2). Study design was variable, consisting of randomised control trials (n=1), retrospective cohort studies (n=9), case series (n=1), and experimental trials (n=13). Ten focussed on the risk of HCW transmission while looking after ill patients, of these, five were published after November 2019. Included studies addressed one of two broad themes, the relative dispersion distance of aerosols by AGP, or infection amongst HCW caring for patient with coronavirus illnesses. Overall, the quality of the included studies for our conclusion of HCW transmission was moderate to poor (Table 2). The studies accepted for quantitative analysis are listed in Appendix 1.
Dispersion distances
A total of fourteen studies investigated the relative dispersion distances of pathogens using various models. Two experimental studies12, 13 investigated the spread of pathogens when using HFNO or NIV. Leung et al12 found no significant difference in dispersion distance of gram-negative bacteria between HFNO at 60 L/min and a conventional Hudson mask at 8.6 L/min (average) on Petri dishes placed at 0.4 m and 1.5 m from the patient’s nose, and air samples at three locations greater than 1 m from the patient after 1 hour. Li et al13 found only nebulised medication, and not NIV, resulted in significant aerosolisation of respiratory viruses or bacteria, measured using SKC Biosamplers (Philadelphia, USA) placed at 1 m and 2 m from the patient.
Loh et al14 conducted an experiment in five healthy subjects who gargled coloured dye and were asked to cough with and without HFNO. Mean (SD) distance of droplet dispersion was 2.48 m (1.03) without HFNO and 2.91 m (1.09) with HFNO. Kotoda et al15 investigated the relative spread of thickened water or yeast solution on a manikin with HFNO at 60 L/min. Dispersion spread was limited to within 60 cm of the manikin’s face. Simonds et al16 characterised the droplet and aerosol dispersion during NIV, oxygen therapy, nebuliser therapy, and chest physiotherapy with 44 patients. They concluded that NIV, chest physiotherapy, and oxygen therapy are droplet-generating but not aerosolising procedures, recommending studies correlate droplet size with risk of viral transmission. Iwashayna et al17 measured aerosol production in four healthy volunteers with room air, nasal cannulae (6 L/min), non-rebreather face mask (15 L/min) and HFNO at 30 L/min and 60 L/min. Aerosol sampling occurred over three minutes at a location 10 cm from the volunteer’s face, and at the bedrail adjacent to the volunteer’s head. There was no difference in relative aerosolisation between these oxygen delivery methods.
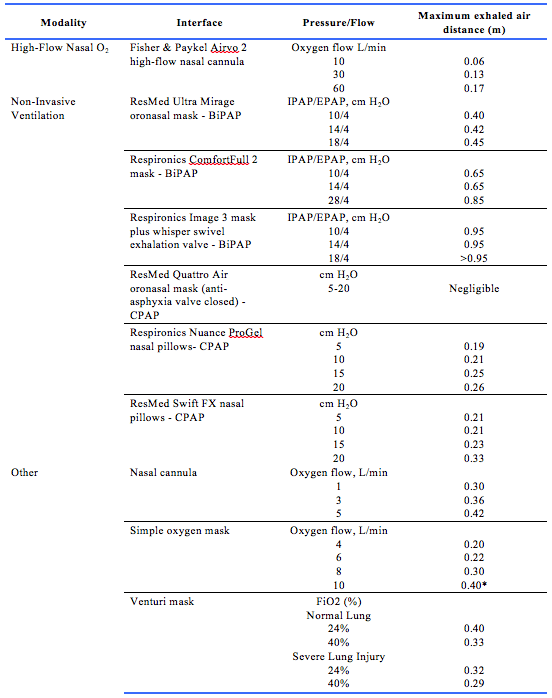
Between 2006 and 2020, Hui et al characterised the relative exhaled air dispersion of a number of procedures using a laser smoke visualisation method, calculating spread in the medial sagittal plane.18-25 These studies were all conducted in a negative pressure room, using a high-fidelity human patient simulator that represented a 70 kg adult male lying on a hospital bed inclined at 45°. The results of these studies are summarised in Table 3.
HCW Transmission
Ten non-randomised studies commented on the transmission of a range of Coronaviruses to HCW during hospital care of infected patients. Seven of these studies, including 1112 HCW caring for 78 patients, were included for quantitative analysis. Of the 3 non-included studies, two (Han et al, Rello et al)26, 27 reported no infections in 145 patients requiring NIV, but the number of HCW was not reported. The 3rd study by Cheng et al28 reported no infections in 413 HCW caring for 42 patients but did not specify which oxygen therapy HCW were exposed to. A forest plot of the outcome data is shown in Fig. 2.
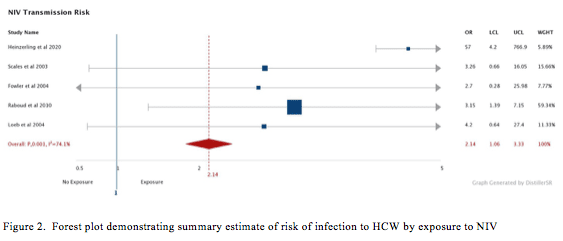
All 7 studies included for quantitative analysis commented on HCW being exposed to NIV (Appendix 1). A total of 276 were exposed, of which 20 were infected. Five cohort studies included for summary estimated by random effects modelling demonstrated an increased risk of HCW transmission during NIV.29-32 A summary estimate yielded a RR of 2.06 (95% CI 1.30 to 3.11, p<0.01) but with significant statistical heterogeneity (I2=74.1%) (Fig. 2). Two cohort studies33, 34 reported 144 HCW who were exposed to NIV during the care of 21 patients, none of whom were infected with the virus. These studies did not report HCW not exposed to NIV and were not included in the summary estimate. Two cohort studies30, 31 commented on HCW exposure to HFNO and the risk of infection. A total of 118 HCW caring for 26 patients with HFN were included, of which three were infected. A summary estimate yielded an insignificant RR of 0.61 (95% CI 0.19 to 1.99, I2=53.2%, p=0.8, Fisher’s Exact Test).
Comparison of published guidelines on the use of HFNO and NIV in COVID-19
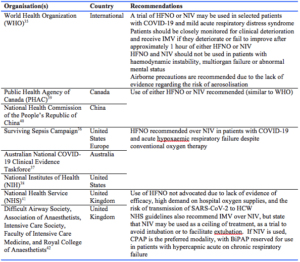
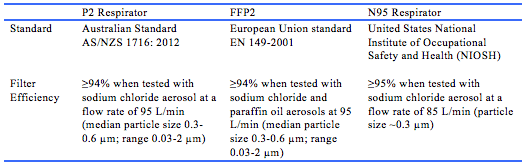
A targeted search of available national and international guidelines found guidelines from eight government organisations and medical societies/collaboratives that made recommendations on the use of HFNO and/or NIV in the treatment of COVID-19.35-42 These recommendations are summarised in Appendix 2. The World Health Organization (WHO) recommends that a trial of HFNO or NIV may be used in selected patients with COVID-19 and mild acute respiratory distress syndrome, with similar recommendations by the Public Health Agency of Canada and the National Health Commission of the People’s Republic of China.35, 39, 40 The Surviving Sepsis Campaign, Australian National COVID-19 Clinical Evidence Taskforce, and United States National Institutes of Health (NIH) recommend HFNO over NIV in patients with COVID-19 and acute hypoxaemic respiratory failure despite conventional oxygen therapy.36-38 In contrast, the United Kingdom National Health Service (NHS) and guidelines from the Difficult Airway Society, Association of Anaesthetists, Intensive Care Society, Faculty of Intensive Care Medicine, and Royal College of Anaesthetists do not advocate the use of HFNO in COVID-19.41, 42 The differences in the recommendations are mainly due to the lack of certainty regarding the efficacy and risk of viral transmission of HFNO and NIV. In order to mitigate the risk of viral transmission to HCW, the general recommendation from available guidelines is to use airborne PPE (including P2/N95 respirator; see Table 4 for standards) and perform AGPs in a negative pressure room. Where negative pressure rooms are not available, single rooms or open spaces with cohorting of patients may be appropriate.
DISCUSSION
In this systematic review of 24 studies, the published evidence was generally of poor quality, mainly comprising experimental studies of droplet size/dispersion distance and epidemiological studies of HCW infection. Nevertheless, whilst conclusions regarding the HCW safety of HFNO and NIV in COVID-19 cannot be made with a high level of certainty, available evidence did provide some indication of the level of transmission risk.
Studies examining droplet size and/or droplet dispersion distance as measures of potential for airborne transmission showed that HFNO and NIV mainly produce large droplets and may not be major AGP. This is an important finding, as AGP place HCW at a considerably higher risk of infection compared to large droplet-generating procedures or actions (such as coughing). Risk of infection from large droplets may be mitigated by adherence to droplet precautions and adequate distancing from the patient. Data from laser smoke visualisation studies using human patient simulators (summarised in Table 3) showed that droplet dispersion was <1 m for both HFNO and NIV, with similar findings for other oxygen delivery modalities (including low-flow nasal prongs, non-rebreather mask, and Venturi mask), suggesting that these procedures are likely to be large droplet- generating rather than small droplet (aerosol) generating. These findings for HFNO droplet dispersion were supported by the in vivo study conducted by Leung et al12 which showed no difference in surface or airborne bacteria contamination in patients with bacterial pneumonia when using either HFNO or oxygen mask. Similarly, Simonds et al16 showed under in vivo conditions that, depending on the type of mask/circuit used, NIV may generate an increased number of large droplets (diameter >10 µm), which are likely to fall from suspension within 1 m of the patient, but does not generate small droplets.
Evidence from meta-analysis of studies on HCW transmission, whilst generally of low quality, suggests that NIV (RR 2.02, 95% CI 1.30 to 3.11, I2=74.1%, p<0.01), but not HFNO (RR 0.61, 95% CI 0.19 to 1.99, I2=53.2%, p=0.8), may increase the risk of coronavirus transmission to HCW. Of the seven studies included for quantitative analysis (Appendix 1), only two studies were in COVID-19 while the other five studies were in SARS, which shares many similarities with COVID-19. In both COVID-19 studies, HCW wore only standard droplet PPE (with a surgical mask) rather than the recommended P2/N95 respirator. No cases of HCW infection with SARS-CoV-2 were reported by Ng et al34 after exposure to NIV, and HCW infected after exposure to HFNO and/or NIV in the Heinzerling et al31 study were reported to have come within 6 feet (~1.8 m) of the index patient. Similarly, there was variable use of appropriate PPE in the studies assessing HCW infection with SARS-CoV-1. Hence, no conclusion can be made about the potential for airborne transmission of SARS-CoV-2 or the safety of HFNO and NIV when appropriate airborne PPE is worn. However, evidence of effectiveness of respirators is poor (based on four systematic reviews from SARS outbreak and the 2009 H1N1 pandemic) due to poorly controlled studies, and there is no strong evidence to suggest that the use of masks/respirators alone are effective in reducing the risk of viral transmission.43, 45
Limitations
Considering the rapidly changing nature of COVID-19, a review protocol was not submitted for registration. The quality of evidence available for inclusion in this review was moderate to poor. Most studies were at risk of selection bias and incomplete data. There was a lack of randomised trials and many studies included here were not specifically designed to assess the relative nosocomial infection risk of HFNO and NIV. The main limitation of the droplet dispersion studies was that none specifically measured SARS-CoV-2. The human patient simulator studies provide useful evidence on aerosol-generating capacity of HFNO and NIV but did not take into account droplet-generating actions, such as coughing or sneezing, which may increase dispersion distance (as shown by Loh et al14), and were conducted using specific parameters that may not be applicable in all patients. Whilst the included studies measuring viral or bacterial dispersion during HFNO or NIV use provide a more direct measure of airborne transmission potential, it is difficult to draw conclusions related to COVID-19 due to the variable ability of pathogens to remain viable when aerosolised. These limitations of the existing studies are consistent with those identified in the recent review by Agarwal et al.6 The main limitations of studies included for quantitative analysis were the inadequate reporting of PPE used, inconsistent uptake of PPE within and between studies, and variable interpretations of ‘standard precautions’. Improved uptake of appropriate PPE, technological improvements in oxygen delivery devices (e.g., decreased leakiness of circuits), increased use of viral filters, and increased availability of negative pressure rooms mean that risk to HCW in the current COVID-19 pandemic may be lower than that suggested by studies conducted during the SARS epidemic.
Implications of Findings
Our systematic review adds to the findings of the review by Agarwal et al6 by including both HFNO and NIV, and examining studies of HCW transmission of SARS-CoV-2 and other coronaviruses. Importantly, we found that that NIV but not HFNO may increase the risk of HCW infection, although this risk may be mitigated by adherence to appropriate PPE precautions and remaining an adequate distance from the patient. As similarly concluded by Agarwal et al6, the limited available evidence supports the need for further high-quality studies investigating the potential for airborne transmission of SARS-CoV-2 and epidemiological studies on HCW infection. Additionally, further research on the characteristics of the SARS-CoV-2 virus and the pathophysiology of SARS-CoV-2 infection is required. An understanding of the infectious dose (amount of pathogen required to cause infection), the relative infective potential in the lower airways compared to the upper airways, and host defences against SARS-CoV-2 infection will allow the risk to HCW to be further elucidated. These factors have implications not only in further characterising the virus but also in determining aerosol risk. The use of airborne precautions and negative pressure rooms when using HFNO or NIV in COVID-19 is appropriate and recommended in international guidelines. Robust reporting of HCW exposures and infections and further high-quality studies are needed to evaluate the effectiveness of the risk reduction measures and identify areas for improvement.
CONCLUSION
The evidence regarding the safety of HFNO and NIV for HCW when used in COVID-19 patients is generally of poor quality, which is reflected in the strikingly different recommendations in international guidelines. However, the available evidence suggests that NIV, but not HFNO, may increase the risk of HCW infection. Additionally, HFNO and NIV are mainly large droplet-generating rather than AGPs, meaning that risk to HCW is probably low provided that appropriate risk reduction measures are implemented, including airborne PPE, utilisation of negative pressure rooms, and minimising close contact with COVID-19 patients. International guidelines should be adapted as additional evidence becomes available to ensure that the optimal balance between effective treatment of COVID-19 patients and HCW safety is achieved.
Provenance: Externally reviewed
Ethics: Not required
Disclosures: No disclosures made
Acknowledgements: We thank the staff of the Fiona Stanley Hospital Intensive Care Unit for their assistance and guidance whilst undertaking this review.
Corresponding author: Mr Toby Price, Faculty of Health and Medical Sciences, The University of Western Australia, 35 Stirling Hwy, Crawley WA 6009, Australia. Toby.Price@health.wa.gov.au. The authors wish to state that HN and TP contributed equally to all aspects of this work.