Introduction
Superficial fungal infections are estimated to affect one billion people globally each year.1 Dermatophytes are a group of keratinophilic filamentous fungi responsible for a significant proportion of fungal skin, hair and nail infections.2 These include nine genera, of which Trichophyton, Microsporum and Epidermophyton most commonly infect humans.3 The anthropophilic species T. rubrum is responsible for most infections world-wide. However, significant geographic and demographic variation exists, with a recent emerging predominance of zoophilic species M. canis and T. mentagrophytes,4 in some areas likely due to increased contact between animals and humans. Additionally, there has been unprecedented growth of dermatophytoses in developing countries, including increases in chronic and relapsing cases associated with significant morbidity, financial burden and impaired quality of life.5
Concern has been raised regarding the development of dermatophyte resistance to existing antifungal agents, including azoles and allylamines, contributing to therapeutic failure. Although already a major public health threat in endemic areas such as India, reports of dermatophyte resistance to terbinafine have increased globally in recent years including in Japan, European countries, Iran, Mexico and the US.6-11 Whilst previously limited to T. rubrum, resistance is now being reported in T. mentagrophytes and T. interdigitale.12Mechanisms for terbinafine and azole resistance include alteration of drug targets, drug efflux and biofilm formation.6,13 Selective pressure for resistant dermatophyte strains is multi-factorial, and may be due to poor compliance, sub-inhibitory doses, widespread self-treatment and steroid misuse.14 Moreover, cellular drug targets are limited and overlapping, for instance, allylamines, azoles, morpholines and thiocarbamates all inhibit ergosterol biosynthesis, albeit through different enzyme targets.14 This has stimulated the search for new formulations to enhance current drug effectiveness, or the development of novel antifungal compounds.15
Plants possess natural defence systems against external microbial pathogens, and serve as a limitless source of antimicrobial compounds.16 Several plants have been utilised historically by native populations to treat fungal infections, particularly in Latin America, Asia and Africa. For example, a survey conducted among 34 Ayurvedic practitioners in Sri Lanka identified 165 plants used for skin diseases, including neem (Azadirachta indica A.Juss.) and turmeric (Curcuma longa L.).17 Many of these species have been scientifically investigated in vitro, and are often chosen based on their uses in traditional medicine.
The aim of this review is to summarise the current scientific evidence in vitro and in vivo for plant based natural therapies for dermatophyte infections.
Methods
A comprehensive search of the literature was conducted across databases PubMed, Embase, and ScienceDirect from their inception to January 2021. Keywords included ‘dermatophyte’ or ‘anti-dermatophytic’ or ‘antifungal’, combined with ‘natural’, ‘ethnomedical’, ‘plant’, ‘botanical’, ‘treatment’ or ‘remedy’. A comprehensive list of plants was extracted from the initial search, and databases searched again using each individual plant name and ‘dermatophyte’, to identify all relevant studies. In vitro studies included described methods of plant extraction and anti-microbial susceptibility testing methods. Minimum inhibitory concentration (MIC) and phytochemical composition was reported if investigated within the in vitro studies selected. In vivo studies included both animal models and clinical trials. One hundred and ten studies of 70 plant extracts were selected after the exclusion of duplicates and irrelevant articles by AM. Concentrations of compounds are reported here using the same units as in the cited study.
Results 1: Plants with in vitro anti-dermatophytic activity
Seventy plants demonstrated in vitro anti-dermatophytic activity (Appendix 1: click HERE). Interpretation of MIC values is difficult due to variations in methodology and no standardised minimum breakpoint concentration deemed to indicate effectiveness against dermatophytes. Additionally, comparison between studies of the same plant extract frequently reveals discrepancies of reported MIC or diameter inhibition zone values. Of the 70 plant extracts included in this review, 25 had more than two dedicated in vitro studies, but very few revealed consistent data. Variations in methodology include inoculum size and preparation, incubation duration and time, criterion used for MIC determination, differences in solubility of phytochemicals in solvents used, environmental growth conditions affecting compound composition, and fungal sensitivity, all of which all significantly influence outcomes. The use of negative and positive controls would be helpful in determining usefulness in clinical application, but these are not always applied. Of thirty-six plant families, Lamiaceae (mints) and Myrtaceae (Myrtle) comprised the most, (10 and 8 plants respectively). Notable plants with the highest antifungal activity as demon-strated by an MIC of < 50 μg ml-1 were Azadirachta indica A. Juss, Piper betle L., Vitis vinifera L., Terminalia chebula Retz., Lawsonia inermis L., Ocimum sanctum L., Libidibia ferrea (Mart. Ex Tul.) L.P. Queiroz, Mimosa tenuiflora (Willd.) Poir, Foeniculum vulgare Mill, Anagallis arvensis L.and Piper regnellii (Mia.) C. DC. Across studies of plants with the lowest MIC values, many common constituents are identified. For instance, essential oil of P. betle comprising primarily eugenolreported an MIC of 0.2 – 0.4 μgml-1 against Trichophyton and Microsporum species.18 Eugenol was also the main component of O. sanctum (MIC 0.4 – 0.8 μg ml-1)18 and Syzygium aromaticum L.(MIC 160 μg ml-1)19. Several other studies showed a weak to moderate inhibitory effect, with MICs ranging up to 160 mg ml-1 reported as having antifungal activity. The impact of methodological variations is highlighted by a 2004 study of T. chebula aqueous extracts, which yielded an MIC against T. mentagrophytes of 600 μg ml-1, whereas a 2013 study using lyophilised ethanolic extract yielded an MIC of 3.125 μg ml-1.20
In general, plant extracts were less effective in vitro than commercial antifungal drugs. However, isolated phytochemicals showed greater inhibitory growth effects than that of the plant extract alone, and sometimes had efficacy comparable or greater than their positive controls. For example, macrocarpal C isolated from Eucalyptus globulus Labill.had an MIC of 1.95 μg ml-1, similar to terbinafine (0.625 µg ml-1 ) and nystatin (1.25 µg ml-1).21 Isolated thymoquinone from black cumin (Nigella sativa L.) had an MIC of 0.125 – 0.25 mg ml-1 compared to the plain ether extract (40 mg ml-1).22 Other examples include terpinen-4-ol from tea tree oil (Melaleuca alternifolia (Maiden & Betche) Cheel), limonene and methyleugenol from Thapsia villosa L., and thymol from Thymus pulegioides L.23,24,25
Synergistic activity is seen when plant extracts are used in combination with existing antifungal drugs. In a study by Khan and Ahmad (2011), the addition of cinnamaldehyde to fluconazole reduced the MIC 8-fold against T. rubrum from 200 μg ml-1 to 40 μg ml-1.19 Similarly, the addition of eugenol or Cinnamomum verum J. Preslreduced the MIC of fluconazole to 80 μg ml-1.19 Synergy was also observed against Aspergillus species suggesting broad spectrum antifungal activity.19 Strong synergism is also noted in studies of Mentha piperita L. and its components menthone and menthol with itraconazole against T. mentagrophytes,26as well as citronellol and geraniol from Pelargonium graveolens L’Hér combined with ketoconazole against Trichophyton spp.27
Some plant extracts are also reported to be effective against resistant strains of dermatophytes. The extract of Pothomorphe umbellata (L.) yielded an MIC of 78 μg ml-1 against T. rubrum containing genes associated with multi-drug resistance.28 Cinnamaldehyde isolated from C. verum was also effective against fluconazole and itraconazole resistant strains of dermatophytes with an MIC of 40-80 μgml-1.19 Essential oil of Cymbopogon citratus (DC.) Stapf and citral also generated inhibited zones of diameter 24.7 – 32.6 mm against azole resistant strains of T. rubrum.29 The proposed mechanisms and utility of such plant extracts in overcoming drug-resistant fungi are discussed below.
Results 2: Plants with in vivo anti-dermatophytic activity
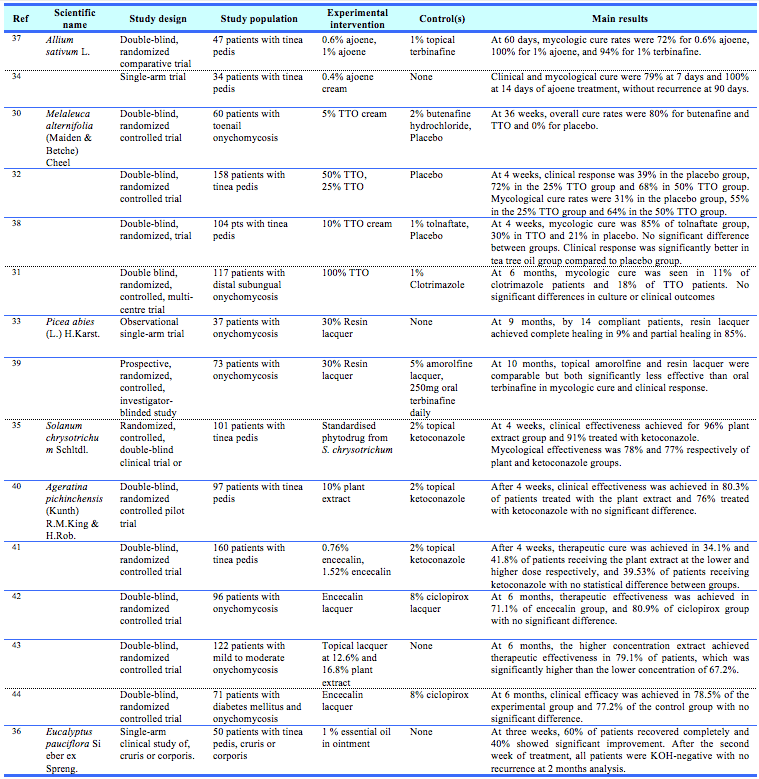
Twenty-one plant extracts were evaluated using in vivo bioassays. Of these, only 6 plant preparations have been tested in clinical trials, summarised in Table 1. Tea tree oil was evaluated in four randomised controlled trials, of which two demonstrated significantly better outcomes compared to placebo, and were comparable to commercial antifungal preparations including topical butenafine and topical clotrimazole.30, 31 However, one randomized double-blind trial randomizing 104 patients with tinea pedis to 1% tolnaftate, placebo or 10% tea tree oil cream showed significantly lower mycological cure rates with tea tree oil (30%) compared to tolnaftate (85%).32 Nonetheless, tea tree oil was significantly better than placebo.32 Isolated encecalin from snake root (Ageratina pichinchensis (Kunth) R.M.King & H.Rob.) was evaluated in five small randomised, double-blind controlled trials in patients with tinea pedis and onychomycosis and showed comparability to topical ketoconazole and ciclopirox respectively. In patients with onychomycosis, topical lacquer made from norway spruce (Picea abies (L.) H.Karst.)was as effective as 5% amorolfine (13% vs 5% cure, respectively), but significantly less effective than oral terbinafine in achieving mycological cure (56% at 10 months).33 Garlic (Allium sativum L.), snow gum (Eucalyptus pauciflora Sieber ex Spreng.) and Devil’s fig (Solanum chrysotrichum Schltdl.) have also been tested in clinically but are preliminary and of small size.34-36
The remaining plant extracts tested in vivo were animal studies (Appendix 2: click HERE), most commonly in guinea pigs, but also in mice, sheep and cattle. Five studies used anthropophilic dermatophyte species. Studies consistently demonstrated efficacy through both clinical cure and mycological cure, comparable to pharmacological preparations. Effects were dose-dependent with negligible dermal irritation. Synergistic activity was also observed in a study of twenty-four cattle with T. verrucosum infections exposed to enilconazole, N. sativa extract, or combined treatment of enilconazole and plant extract. Combined treatment resulted in healing of all 8 cattle, whereas single agent enilconazole or N. sativa treatment resulted in healing in 5 and 6 animals respectively.22
Table 1. Summary of clinical trials of anti-dermophytic plants
Mechanism of action of natural anti-dermatophytic compounds
The anti-dermatophytic mechanism of action in plants is multi-modal and includes disruption to cell membranes and cell wall synthesis as well as inhibition of hyphal growth and spore germination. These actions are mediated by abundant active plant compounds, of which phytochemical screening indicate the presence of phenolic compounds, terpenoids, terpenes, alkaloids, xanthones and glycosides including saponins, and have been well-characterised.45
Phenolic compounds are found abundantly in plants and include phenols, flavonoids, tannins, quinones, coumarins and phenolic acids. They are extractable by water, methanol or acetate.46 Gallic acid has been shown to inhibit ergosterol biosynthesis by reducing activity of sterol-14α-demethylase P450 and squalene epoxidase in T. rubrum.47 Ergosterol content in the fungal membrane was significantly decreased at all tested concentrations of gallic acid (between 43.75 and 83.33 μg ml-1).47 Correlation between content of phenolic compounds and antifungal activity is observed in a study of Vitis vinifera L., where grape varieties Alphonse-Lavallee containing the highest flavon-3-ol content also exhibited the highest antifungal activity.48 Tannins, found in many plants including Mimosa tenuiflora Benth., Hypericum perforatum L., Moringa oleifera Lam.and Urtica dioica L., inhibit cell wall synthesis through formation of irreversible complexes with proteins.49
Saponins have been previously reported to cause leakage of protein and enzymes from the cell by complexing with sterols in the cell membrane.50 They were the most effective anti-dermatophytic compound in Polyscias fulva (Hiern) Harms.51 Saponins have also been studied in the context of Candida which have also concluded alteration to cell membranes as the main mechanism.52 Increased permeability to propidium iodide for Candida in a study by Pinto (2009) suggests that there is a primary lesion of the cell membrane leading to cell death.53
Terpenoid phenols also exhibit strong broad-spectrum antifungal activity. They include eugenol, which was reported as a main constituent of thirteen antifungal plants, as well as thymol and cavacrol found in Thymus vulgaris L., T. pulegioides and Origanum vulgare L. Essential oil of O. gratissimum containing 57.8% eugenol, viewed under transmission electron microscopy, caused morphological changes to dermatophyte hyphae, with damages to cell wall and membranes and expansion of endoplasmic reticulum.54 Flow cytometric studies with lemon thyme and active compounds thymol and cavacrol, showed lesion formation in the cytoplasmic membrane and a reduction on ergosterol content.25 Suggested mechanisms of cell membrane disruption and cell death occur include calcium stress (increased cytosolic calcium leading to loss of cell viability) and inhibition of the Target of Rapamyci n (TOR) pathway.55
Interference of hyphal growth and spore germination contributes to the anti-dermatophytic action of plant extracts. Extract of Tetradenia riparia (Hochst.) showed strong inhibition and irregular growth pattern of hyphae through scanning electron microscopy.56 Additionally, essential oil volatiles of Agathosma betulina (P.J.Bergius) Pillansinhibited spore production of T. rubrum.57Further subcultures of mycelia did not result in growth suggesting strong fungicidal action due to irreversible damage.57 This is significant, as spores are asexual reproductive structures which can initiate skin infections and are responsible for transmission in the community. The hydroalcoholic extract of Piper regnellii was further able to inhibit spore germination in a dose-dependent manner, and 7.8 μg ml-1 inhibited T. rubrum spore generation by 100%.57 Nail fragments that were saturated and immersed in P. regnellii extract at a concentration of 1.2 mg ml-1 and inoculated with spore suspension did not experience mycelial growth, whereas the nail fragments not exposed to the plant extract had vigorous growth.58
Other compounds investigated for their mechanism of action include monoterpenes, terpenoids generally and sesquiterpenoids. Macrocarpal C, a sesquiterpenoid isolated from E. globulus, waseffective against T. mentagrophytes though several mechanisms including increased membrane permeability, increased intracellular reactive oxygen species and induction of apoptosis through DNA fragmentation.21 Monoterpenes found abundantly in essential oils include geranial, geraniol, citral and citronellol are suggested to mediate their antifungal activity through inhibition of spore production, and binding of ergosterol resulting in fungal cell membrane destabilisation.57, 59
In vivo anti-inflammatory and immunomodulatory actions of anti-dermatophytic compounds
In addition to direct anti-dermatophytic effects, plant compounds have anti-inflammatory and immunomodulatory effects, which can enhance treatment effects in vivo. Extract of Salacia senegalensis (Lam.) DC had inhibitory effects against soybean 5-lipoxygenase, an important enzyme in the inflammatory and oxidative response in humans.60 Immunomodulation has also been proposed in studies where in vivo anti-dermatophyticefficacy were observed, but in vitro efficacy is not, by enhancement of immune self-resolution. For instance, in guinea-pigs with induced M. canis dermatophytosis, U. dioica extract at 100 mg ml-1 concentration did not achieve in vitro activity, but an in vivo assay demonstrated efficacy comparable to terbinafine, and was significantly better than negative control.61 In another in vivo study of Astragalus verus Olivierin T. verrucosum infected guinea-pigs, 40% concentration was comparable to positive control terbinafine. However, lesions improved in the negative control at 13 days suggesting resolution is partially contributed by immune response. Immunomodulatory mechanisms of Astralagus were studied by Guo et al., (2016), showing dose-dependent inhibition of over-production of cytokines TNF-α, IL-1β, IL-6 and IFN-γ in LPS-stimulated macrophages.62
Current limitations in research, and future prospects
Despite numerous in vitro studies demonstrating antifungal activity, only a small proportion utilise rigorous methodology as required for robust evidence. Cos et al. (2006) outline a set of parameters and efficacy criteria for high quality evaluation of anti-infective potentials of natural products.63 These include use of reference strains, in vitro models on the whole organism, cytotoxicity testing, dose ranges reflecting a practical inhibitory concentration, and use of positive and negative controls. An endpoint criteria for all anti-infective bioassays was recommended as having usual IC50 values (the minimum drug concentration inhibiting 50% of fungal growth), below 100 μg ml-1,and below 25 μM for pure compounds.63 Most anti-fungal studies have reported MIC (the lowest drug concentration inhibiting visible fungal growth). However, endpoint criteria for antifungal efficacy and cytotoxicity assays are rarely stated in in vitro studies. Considering this, the number of plants having significant anti-dermatophytic properties may be exaggerated. Commonly utilised antimicrobial susceptibility testing methods include standardised protocols by CLSI or EUCAST, whilst some studies use agar well diffusion methods or poisoned food techniques.64
In vitro efficacy also may not translate into in vivo efficacy, particularly in humans. Many fungal, host factors such as immunosuppression and compliance, suboptimal absorption and penetration are all clinically relevant.6 Importantly, penetration of antifungals into the stratum corneum and its persistence is important in achieving cure. Most in vivo data is based on animal models rather than clinical trials, which may undermine clinical applicability given differences in virulence factors and immune responses.65 For example, T. mentagrophytes, a zoophilic species,tends to cause an acute inflammatory response whereas T. rubrum, an anthropophilic species,causes minimal inflammation.65 Clinical trials show some encouraging results but generally constitute insubstantial evidence, given limitations of small sample size and methodological flaws.
Possible adverse outcomes of plant therapies such as contact dermatitis17 should also be considered, in case of indiscriminate use for commercial purposes. For example, plants belonging to Rutaceae, the citrus family, or Moraceae, can cause phytophotodermatitis owing to photosensitising compounds furocoumarins and psoralens.66 This can be clinically overlooked if the extract is not applied to sun-exposed areas, or are only momentarily used to prevent colonisation.67 Alternatively, these undesirable components can be removed, such as furocoumarin-free extracts of bergamot oil, which retained their anti-dermatophytic activity in in vitro studies. 68 In most animal and human studies of anti-dermatophytic plants, minimal to no serious adverse effects have been reported from topical application, noting small sample sizes. For example, in one study evaluating 5% Melaleuca alternifolia oil in cream for toenail onychomycoses, four of 40 patients reported only mild inflammation.30 Nonetheless, the risk of adverse cutaneous effects to topical plant extracts in general should be acknowledged. In one multi-centre study of 1274 patients using topical botanical products, 11% of patients reported adverse cutaneous reactions based on a questionnaire, and 16% showed positive reactions when tested in a botanical series.69 In studies using cell cytotoxicity assays, optimistic results may be seen with minimal reports of toxicity. For example, in a study evaluating S. senegalensis leaves, human epidermal HaCaT keratinocytes experienced no detrimental effects at concentrations displaying anti-dermatophytic and anti-inflammatory properties. Cytotoxic activity was also evaluated for 2.5 μL ml-1 A. major oil, β-ocimene and α-pinene using a haemolytic activity assay and found 10%, 10% and 15% haemolysis respectively.70 This shows promise for natural agents with high antifungal activity and low toxicity. However, significantly more cytotoxic studies are required to establish stronger safety evidence for plant-based treatments.
Antifungal resistance is a key global health problem and innovation and testing of natural products holds enormous potential. However, the mechanisms of plant extracts responsible for activity against drug resistance are not well investigated. Inhibition of multidrug (MDR) efflux pumps, and normal cell communication (quorum sensing) have been described as some of the key mechanisms against bacterial MDR pathogens.71 Synergy between diverse plant constituents is suggested to play a key role in the effectiveness of herbal medicine in anti-microbial resistance.72 As previously discussed, the anti-fungal mode of action of plants is due to numerous potential bioactive compounds and therefore multiple targets. Unlike agents with single constituents and targets, the presumed chances of development of resistance are lower with multiple targets. However, there is little research on whether fungi or other microbes will develop resistance against plants similar to the mechanisms of resistance evolved against current pharmaceuticals. Scientific evaluation of complex synergistic interactions is furthermore a likely challenging and costly process, without fully developed technology to study these mechanisms.71
Significant synergistic antifungal activity with conventional antifungals highlights the potential for combined drug therapy. For instance, the combination of essential oils with topical drugs can enhance penetration to deeper skin layers via abundant low molecular weight terpenes.73 Combined therapy may also reduce therapeutic doses, improving side effect profiles, mycological clearance and development of resistance.74
Despite the comprehensive search strategy used here it is likely many plant extracts with antifungal potential were not located, due to the innumerable diversity of the plant kingdom and ethnomedical studies not published in electronic databases.
Conclusions
Superficial fungal infections are highly prevalent and the prevalence of chronic and resistant cases is increasing, highlighting the need for novel anti-dermatophytic compounds. Based on numerous in vitro studies, many plant extracts demonstrate anti-dermatophytic efficacy. The plant extracts or isolated compounds have significant potential to be used in many contexts: directly applied alone – particularly in third world countries without readily accessible commercial antifungal drugs; incorporated into existing antifungal drugs for synergistic action; or further explored as novel antifungal drugs. Despite this, very few of these studies have had in vivo testing or clinical trials to assess their safety and effectiveness, although those conducted so far have been promising. Further in vivo studies will enable exploration and investment into new clinically effective anti-dermatophytic compounds, which is essential in the face of emerging dermatophyte resistance.
Provenance: Externally reviewed
Ethical Approval: Not required
Disclosures: None
Acknowledgements: None
Corresponding Author: Dr Angela Mei, Sir Charles Gairdner Hospital, Nedlands, Perth, WA 6009, Australia. Email: angela.mei@health.wa.gov.au









